
Home / Atomic Orbitals / Shapes of the 3d orbitals in 3D. The molecule formed due to this hybridisation is tetrahedral, with an angle of 109o28. Interactive colour surface representations for the five d orbitals in 3D showing the nodes. Thus the most probable radius obtained from quantum mechanics is identical to the radius calculated by classical mechanics. The molecules possessing sp hybridisation used to have a linear shape with an angle of 180°. s orbitals are spherically symmetric around the nucleus - in each case, like a hollow ball made of rather.
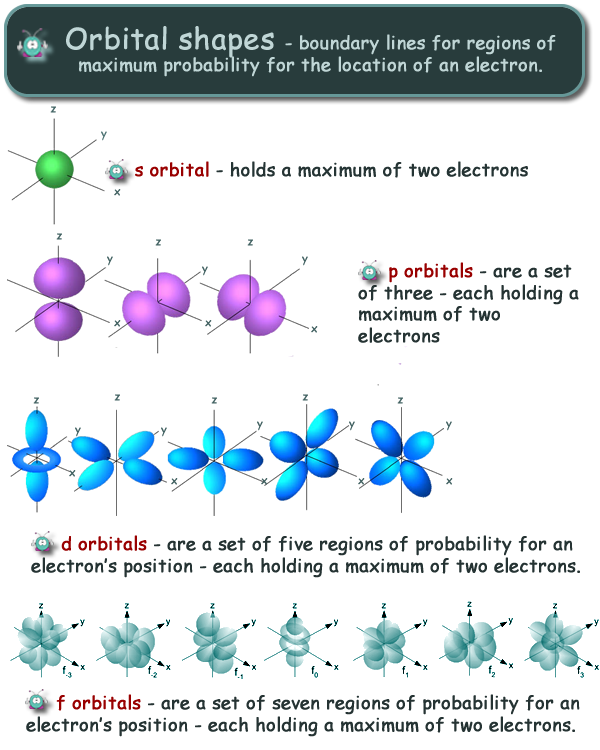
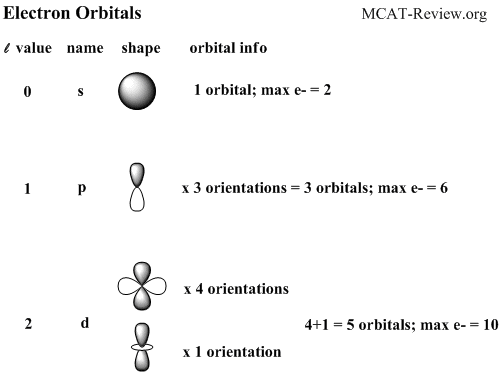
This peak corresponds to the most probable radius for the electron, 52.9 pm, which is exactly the radius predicted by Bohr’s model of the hydrogen atom.įor the hydrogen atom, the peak in the radial probability plot occurs at r = 0.529 Å (52.9 pm), which is exactly the radius calculated by Bohr for the n = 1 orbit. The s tells you about the shape of the orbital. All orbitals with values of n > 1 and ell 0 contain one or more nodes. Orbitals with 0 are s orbitals and are spherically symmetrical, with the greatest probability of finding the electron occurring at the nucleus. For example, the orbitals with (l0) are called s orbitals those with (l1), p orbitals those with (L2), d orbitals, etc. The four chemically important types of atomic orbital correspond to values of 0, 1, 2, and 3. Recall that each value of l is assigned a letter that corresponds to particular orbitals. Because the surface area of each shell increases more rapidly with increasing r than the electron probability density decreases, a plot of electron probability versus r (the radial probability) shows a peak. The shape of an atomic orbital is determined primarily by l, the angular momentum quantum number. (d) If we count the number of dots in each spherical shell, we obtain the total probability of finding the electron at a given value of r. (c) The surface area of each shell, given by 4π r 2, increases rapidly with increasing r. The density of the dots is therefore greatest in the innermost shells of the onion. Objectives define qualitatively what an atomic orbital is, recognize and identify the shapes of atomic orbitals, recall the number of atomic orbitals that. (b) A plot of electron probability density Ψ 2 versus r shows that the electron probability density is greatest at r = 0 and falls off smoothly with increasing r. (a) Imagine dividing the atom’s total volume into very thin concentric shells as shown in the onion drawing. \): Most Probable Radius for the Electron in the Ground State of the Hydrogen Atom.


 0 kommentar(er)
0 kommentar(er)
